 |
 |
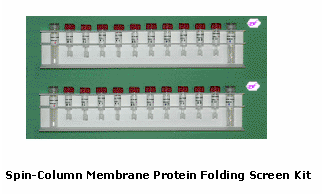
Spin-column Membrane Protein Folding Screen Kit
(Catalog # MFC01-20)
The Spin-column Membrane Protein Folding Screen Kit (catalog # MFC01-20) is specifically designed for folding membrane proteins from urea-solubilized inclusion bodies or other protein aggregates. The kit is composed of 20 protein folding spin columns and Reagents A, B and C. The 20 spin-columns represent 20 different conditions with various detergents and lipids that form micelles and bicelles. The micellar and bicellar environments facilitate folding receptors, ion channels and other membrane proteins. When the active protein is identified, preparative columns with the specific condition (the column number) are available for large-scale preparations of the folded membrane proteins.
|
 |
 |
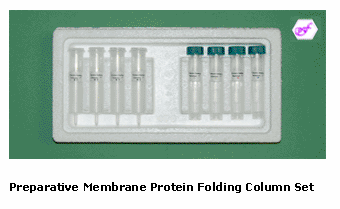
Preparative Membrane Protein Folding Column Sets
The Preparative Membrane Protein Folding Column Sets are used for preparative membrane protein folding after the folding condition has been identified by the Spin-column Membrane Protein Folding Screen Kit (Catalog # MFC01-20). The column number represents the specific folding condition. Each Column Set includes 4 identical preparative protein folding columns and reagents for folding about 5 - 10 mg of urea-solubilized inclusion body proteins.
MFC01 MFC02 MFC03 MFC04 MFC05 MFC06 MFC07 MFC08 MFC09 MFC10
MFC11 MFC12 MFC13 MFC14 MFC15 MFC16 MFC17 MFC18 MFC19 MFC20
References:
- Pelletier DM. Hemoglobin binding protein from Actinobacillus pleuropneumoniae: a novel method for extraction and isolation. McGill University, Montreal Q.C. Graduate thesis, (2007).
- Lee S et al, Cloning, Solubilization, and Characterization of Squalene Synthase from Thermosynechococcus elongatus BP-1, J. Bacteriol., 190: 3808-3816 (2008).
- Giacani L., et al, Transcription of TP0126, Treponema pallidum Putative OmpW Homolog, Is Regulated by the Length of a Homopolymeric Guanosine Repeat, Infection and Immunity 83: 2275-2289 (2015).
- 谢浩,等, 去垢剂在膜蛋白研究中的应用 (Applications of Detergents in Membrane Proteins Research). 生物技术通报 (BIOTECHNOLOGY BULLETIN) 2: (2010).
- 谢浩,等,膜转运蛋白的功能性超表达和纯化(Overexpression and Purification of Membrane Transport Protein with Native Functions) , 中国生物化学与分子生物学报(Chinese Journal of Biochemistry and Molecular Biology, 23 (12):1051 (2007).
- Parveen N. et al, Non-pathogenic Borrelia burgdorferi expressing Treponema pallidum TprK and Tp0435 antigens as a novel approach to evaluate syphilis vaccine. Vaccine, Vol 37, (13)1807 (2019).
- Austin M. Haynes A. M et al, Evaluation of the Protective Ability of the Treponema pallidum subsp. pallidum Tp0126 OmpW Homolog in the Rabbit Model of Syphilis. Infection and Immunity, Volume 87 Issue 8 e00323-19 (2019).
|